Selection and Preparation of Patients
As with other high-tech treatment methods, a crucial condition for the successful application of MRgFUS is the proper selection of patients, with careful consideration of all indications, contraindications, risk/benefit ratio, and prognosis. Since the main experience with MRgFUS in neurology today involves ablative functional interventions for movement disorders, this chapter will primarily focus on the principles of selecting this particular category of patients.
Stages of Patient Selection for Treatment Using MRgFUS
In 2004 T.Ferguson и G.Frydman introduced the term “electronic patient” to describe people who are well-informed about their illness (sometimes even more than their treating doctors) and actively participate in making decisions about their health and medical care, using available information online. A survey of more than 670 patients who sought treatment at our center over 3 years using MRgFUS showed that 79% of them found information independently while searching for new treatment methods through various electronic resources. Usually, such inquiries come from patients with a long history of the disease and an already established diagnosis.
During the initial contact with a patient seeking MRI-guided focused ultrasound, it is necessary to ensure the accuracy of the diagnosis and the adequacy of the ongoing therapy. If conservative therapy was insufficient (in terms of medication dosages, duration of intake) or not entirely correct, the patient is referred for treatment to a neurologist specializing in movement disorders. If, despite adequate therapy, the patient continues to experience severe, disabling symptoms, they proceed to the next stage—an in-depth examination (in-person or online) at a specialized MRI-guided focused ultrasound center.
Features of Using Telemedicine Technologies in Patient Selection
Telemedicine is the use of electronic communication technology for the purpose of providing remote medical care. In the Russian Federation, telemedicine consultations are conducted based on Article 36.2 of the Federal Law dated 29.07.2017 No. 242-FZ. Research on interactive telemedicine technologies in real-time using videoconferencing is actively developing for many neurological diseases. For patients with movement disorders, this is particularly relevant, considering their limited mobility, the progressive nature of the disease, and often the lack of qualified specialists in movement disorders in certain localities ( Schneider, Biglan, 2017; Espay et al., 2019). A sharp increase in attention to the possibilities of telemedicine occurred in connection with the pandemic COVID-19 and the movement restrictions it causes ( Srinivasan et al., 2020).
For the purpose of remote selection of patients for MRgFUS at the neurosurgery clinic of the V.S. Buzaev International Medical Centre (Ufa), we have developed a methodology for conducting neurologist consultations via telemedicine. The corresponding algorithm is presented below.
At the very beginning, the doctor meets with the patient and any accompanying relatives, confirming the patient’s consent for others to be present during the consultation. Complaints and medical history, including family history, are collected (this is significant, for example, in patients with ET). Special attention should be given to the medications being taken, as surgical treatment is the method of choice only for patients refractory to medication therapy. For example, “medication-resistant tremor” is defined as persistent disabling tremor that remains despite attempts to use at least two standard drugs (propranolol, primidone) in sufficient therapeutic doses. It is important to timely identify possible contraindications for the use of MRgFUS (Table 4.1)
Table 1 . Contraindications for the use of MRgFUS in patients with ET and PD
| Contraindications | Explanations and clarifications |
| Inability to perform MRI | Implanted metallic devices incompatible with MRI; weight restrictions; allergy to contrast agents; severe claustrophobia that cannot be overcome. |
| Presence of implants in the brain or on the skull bones | Shunting systems; electrodes; plates; artificial dura mater; clips |
| Obstacles for the passage of ultrasound waves | Presence of areas in the brain, bones, and skin that have increased ultrasonic absorption capacity in the projection of the proposed paths of ultrasound waves (cystic-gliotic changes, changes in the projection of a removed shunting system, scars on the skin surface, etc.) |
| Presence of CNS diseases | Brain tumors, aneurysms, etc. |
| Presence in the medical history of certain diseases during the previous year | Acute cerebrovascular accidents of ischemic or hemorrhagic nature; seizure episodes. |
| Taking anticoagulants | Over the past 2 weeks. |
| Unstable cardiovascular diseases and severe arterial hypertension | Unstable angina, regardless of medication; documented myocardial infarction within the previous 6 months, congestive heart failure with ejection fraction < 40%; unstable ventricular arrhythmia; uncontrolled atrial arrhythmia; diastolic blood pressure > 100 mmHg while taking medication |
| Chronic kidney failure | Severe renal impairment with glomerular filtration rate <30 mL/min/1.72 m 2 and/or undergoing dialysis treatment |
| Infectious disease | Acute phase |
| Presence of certain conditions in medical history | Abnormal bleeding; hemorrhages; coagulopathy. |
| Pregnancy | At any stage. |
| Alcohol and other psychoactive substance abuse | Taking medications affecting the CNS in the past 6 months |
| Contrast agent administration within the previous 24 hours | Diagnostic studies (MRI, CT, ultrasound, X-ray examination) using contrast agents. |
| Increased skull bone density | Bone density coefficient of the skull less than 0.35 (± 0.05) according to CT data |
| Personal characteristics of patients | The patient cannot or does not wish to maintain the required stationary position during treatment (approximately 2 hours) and cannot communicate independently with the staff during therapy. |
Neurological examination using telemedicine technologies
This section presents a minimal set of tests for assessing neurological status, feasible and sufficient to make a conclusion based on the results of an online examination (tests selected based on our 3-year experience). An online consultation takes an average of 60–80 minutes. The patient requires an assistant to help with trials and tests to assess the severity of tremor and other neurological symptoms.
Assessment of cranial nerves During the discussion with the patient about their medical history, speech fluency is assessed, along with any disorders such as dysarthria, dysphonia, or aphasia. Eye movements are evaluated: the patient is asked to look up–down–right–left plus convergence, with a short pause in each position. Facial symmetry is assessed: the patient is asked to close their eyes tightly, raise their eyebrows, puff out their cheeks, and purse their lips. Neck mobility is evaluated: the patient is asked to turn their head right and left, tilt their head right and left. The condition of the tongue, extended from the mouth, is assessed for fasciculations and tremor at rest; the patient is also asked to move the tongue side to side to evaluate the range of motion.
Indirect assessment of muscle strength, muscle rigidity, and bradykinesia Initially, it is necessary to assess the symmetry of muscle mass in the arms and legs. For a basic assessment of the symmetry of volume and movement speed, the patient should be asked to perform a full range of movements with their arms and legs. A bradykinesia test is conducted: clenching and unclenching the fist, pronation-supination of the hand, tapping the toe on the floor.
Assessment of coordination and gait. Evaluated: rapid alternating hand movements – from object to nose; patient’s normal gait, walking on toes and heels; patient’s ability to stand in the Romberg test
Tremor assessment Patient examination at rest: hands should rest relaxed on the armrests of the chair or on the knees in a neutral position between pronation and supination. The patient is asked to subtract 7 from 100 aloud (assessment of the presence or absence of tremor at rest and during this test). Resting tremor is assessed on a scale from 0 to 4 (0 – no tremor; 1 – slight tremor, may be intermittent; 2 – moderate amplitude tremor, may be intermittent; 3 – significant amplitude tremor; 4 – severe disabling tremor)
Examination of the hands while maintaining posture: the patient is asked to extend their arms forward, spreading their fingers. Then the patient is asked to slowly bend their arms at the elbows and perform slow pronation-supination of the forearm, noting changes in tremor and the presence of a kinetic component. To objectify the assessment of postural tremor, the patient can be asked to hold a glass of water alternately in outstretched hands. Postural tremor is assessed on a scale from 0 to 4, similar to the severity gradations of tremor oscillations for resting tremor mentioned above. To identify positional increases in tremor amplitude, it is important to ask the patient to place their hands in different positions (Fig. 4.1)
Examination of hands during movement (detection of kinetic tremor): the patient is asked to touch their nose with their index finger.
You can also ask the patient to alternately lift a glass of water with their right and left hand and bring it to their mouth. Some patients experience an increase in tremor intensity with slight arm loading. In this test, the intentional component of the tremor and dysmetria are evaluated separately. Kinetic tremor is assessed on a scale from 0 to 4, similar to the severity gradations of oscillatory tremors for resting tremor mentioned above.

Letter evaluation In patients with ET, handwriting is characterized by large, sweeping movements and tremors, while in patients with PD, it primarily features micrographia (with or without tremor oscillations)
The study of resting tremor, postural tremor, and kinetic tremor is also conducted in the lower limbs in a lying position. Postural tremor is assessed using the Barré test, and kinetic tremor of the legs is evaluated during the heel-knee test. All three types of tremor are assessed using the aforementioned scale from 0 to 4.
When assessing tremor in the limbs, it is essential to conduct provocative tests to identify the functional component of the tremor.
Via video call, it is also possible to determine axial tremor (tongue tremor at rest and postural, voice tremor, facial muscle tremor, head tremor at rest while lying down and postural while sitting, trunk tremor at rest while lying down and postural while sitting), assess the presence and severity of laterocollis, retrocollis, torticollis. All types of tremor are also assessed on a scale from 0 to 4.
To evaluate the spiral and line drawing test, a standard form is sent to the patient in advance, which they must print out. During the online appointment, the doctor instructs the patient on how to perform these tasks: the drawing is done without resting the hand (Fig. 4.2)
The water pouring test from glass to glass and the water carrying test to the mouth with a teaspoon are also evaluated. All research results are entered by the doctor into the clinical tremor assessment scale ( Clinical Rating Scale for Tremor, CRST), parts 1 and 2.
Selected patients are re-examined by a neurologist on the eve of treatment with MRgFUS.

We conducted a separate study in patients with ET for sub-
claims that the online approach is identical to an in-person examination and does not
yields to it for the purposes of selection for treatment using
MRgFUS. During the online examination, the median total score for
CRST 40.5 [33,2; 46,8], when conducting an assessment in these same
patients at in-person examination – 41 [32; 47]. In Fig. 4.3 presented
comparison of patient group data indicators: statistically significa-
no significant differences detected ( p > 0,005 by the Wilcoxon criterion)
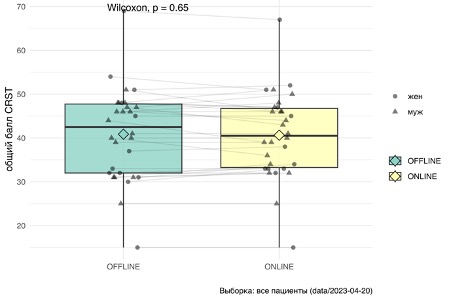
To confirm that the online approach is identical to an in-person examination when assessing tremor on the scale CRST, we conducted a test for equivalence non–inferiority. We used t-criterion for comparing average scores CRST two groups, assuming equal deviations. According to the statistical analysis conducted, no differences were found between the compared groups (p=0.58). In the next stage of the analysis, we accepted a threshold of 5 points as an allowable error and formulated an alternative hypothesis: the medians of the total score on the scale CRST «online” and “offline” examinations differ by more than 5 points (this wording is softer as it allows for not only equivalence but also errors in the range of 0–5 points). As a result, the value obtained was p<0,04 that allows us to reject the alternative hypothesis and conclude that the online examination differs from the in-person examination by no more than 5 points
Thus, it was established that the assessment of ET severity obtained through telemedicine technologies can be an adequate clinical measure for evaluating tremor during remote medical consultations.
Computed tomography
In the second stage of selection, the question of the technical feasibility of performing the MRgFUS procedure is addressed, specifically the assessment of the ultrasound conductivity of the skull bone tissue. For this, the patient is referred for a CT scan of the skull, and the study must be conducted according to special rules.
The MRgFUS console software allows for the calculation of bone density only with strict adherence to a special CT protocol. Compatible protocols with the program General Electric (BONEPLUS), Toshiba (FC30), Canon (FC30). The enhancement filter cannot be used for planning UEO, Contrast. Images are analyzed raw in format FC30 RAW. As a rule, no enhancing filters are applied to CT scans intended for use in MRgFUS procedures. CT is performed with a uniform slice thickness of 0.625–1.25 mm with a 0 mm interval and a resolution of 512 × 512. The series should cover the entire skull, not limited to the target area. The upper slice is always above the skull (in the air), and the last slice reaches the base of the skull. The slices are oriented so that the axial CT images align with the plane AC–PC and perpendicular to the midline.
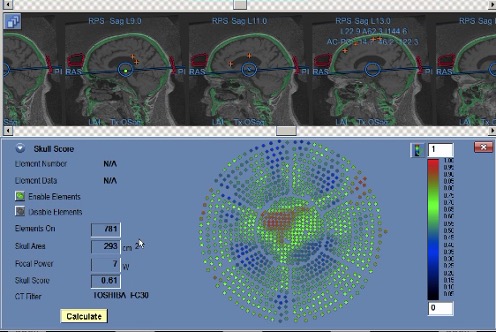
The patient sends their CT images via a secure communication channel, which are then uploaded to the MRgFUS console. The software allows for the calculation of the bone tissue ultrasound conductivity coefficient Scull Score) [423] using the built-in program (Figure 4). The higher the indicator Scull Score, the better the permeability of bone tissue for ultrasound. A coefficient of 0.35 is considered suitable for treatment using MRgFUS: if the bone density is ≥0.35, it is considered technically feasible to perform the procedure.
In principle, even with a dense skull (for example, with “borderline values of the indicator Scull Score) A neurosurgeon has certain technical capabilities during MRgFUS to achieve the necessary temperature at the target point and obtain an acceptable clinical effect by increasing energy, power, frequency, and duration of sonication. However, this is often associated with the occurrence of pain syndrome, which is difficult for patients to endure.
If the bone ultrasound conductivity density coefficient in a patient is below 0.35, treatment is currently considered impossible: even the maximum parameters of MRgFUS cannot guarantee heating at the target point. For such situations, we, together with a team of endocrinologists, have developed a special method to improve bone tissue permeability. Endocrinologists have created a program to change bone density using alendronic acid-based medications taken over 6 months. At the time of writing this monograph, 8 patients in our Center have undergone or are continuing this course of treatment, and two of them successfully underwent MRgFUS after completing the course.
Preparatory stage immediately before performing MRgFUS
To ensure the safety of the MRgFUS procedure, all patients at this stage are required to undergo the following standard examinations (before arriving at the center): complete blood count (no more than 10 days); urinalysis (10 days); blood biochemistry (glucose, protein, creatinine, urea, bilirubin, alanine aminotransferase, aspartate aminotransferase) (10 days); blood test for blood type and Rh factor; blood test for human immunodeficiency virus (6 months) HBs-antigen (6 months), hepatitis C (6 мес) COVID-19 (72 Wassermann reaction (6 months); coagulogram (10 days); electrocardiography (10 days); duplex scanning of brachiocephalic arteries, veins of the lower extremities (10 days); examination by a general practitioner at the place of residence with obtaining a conclusion (10 days). If deviations are detected, patients are additionally consulted by a cardiologist/therapist and endocrinologist at our center to correct somatic pathology and ongoing treatment. In our patient group, this necessity arose in 37% of cases.
For the final decision on the possibility of treatment using MRgFUS, the patient is examined by a neurologist and a neurosurgeon. To objectively assess the condition of patients, a wide range of the standard scales listed below is used.
• Universal scales for any pathologies: MoCA (Montreal Cognitive Assessment – Montreal Cognitive Assessment (MoCA) Nasreddine et al., 2005), HADS (Hospital Anxiety and Depression Scale – Hospital Anxiety and Depression Scale Zigmond, Snaith, 1983), SF-36 (The 36-Item Short Form Health Survey – Short Health Status Survey of 36 Items Ware et al., 1994), MADRS (Montgomery–Åsberg Depression Rating Scale – Montgomery–Åsberg Depression Rating Scale Montgomery, Åsberg, 1979), ESS (Epworth Sleepiness Scale – Epworth Sleepiness Scale) Johns, 1991), Beck Depression Inventory ( Beck et al., 1961).
• Scales for Assessing Tremor Hyperkinesis in Patients with ET and PD. The most commonly used scale for assessing tremor is CRST; at the same time, a general assessment is conducted as well as an assessment for each side of the body separately ( Fahn et al., 1988; Khandhar, Marks, 2007). In ET, it is also advisable to use scales TETRAS (The Essential Tremor Rating Assessment Scale – scale for assessing the severity of essential tremor) Elble, 2016) и QUEST (Quality of Life in Essential Tremor questionnaire – Quality of Life Questionnaire for Essential Tremor Tröster et al., 2005).
• In PD, the “gold standard” is the use of UPDRS (part I–IV and the total score, as well as the separate score for the operated and non-operated sides) ( Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease, 2003), Hoehn and Yahr functional scale Hoehn, Yahr, 1967) и PDQ-39 (Peto et al., 1998).
• In patients with dystonia, depending on the form (CD, myoclonus-dystonia, segmental dystonia), the clinical condition is advisable to determine by UDRS (Unified Dystonia Rating Scale – Unified Dystonia Rating Scale) ( Comella et al., 2003), BFMDRS (Burke–Fahn–Marsden Dystonia Rating Scale – Burke-Fahn-Marsden Dystonia Rating Scale Burke et al., 1985), UMRS (Unified Myoclonus Rating Scale – Unified Myoclonus Rating Scale) ( Frucht et al., 2002), TWSTRS (Toronto Western Spasmodic Torticollis Rating Scale – Western Toronto Spasmodic Torticollis Rating Scale Comella et al., 2015), as well as on CRST (Fahn et al., 1988).
The duration of a neurologist’s examination using scales necessary for a specific patient is 1.5–2 hours. During the joint consultation of a neurologist and a neurosurgeon, in addition to clarifying the indications for MRgFUS, the patient is given a detailed explanation of the treatment stages, sequence of manipulations, possible sensations during their execution, and other questions. Then, an examination by a cardiologist (therapist) of our center is conducted. After completing all the specified procedures, a final decision on the surgery is made collegially.
Preparing a patient for MRgFUS involves discontinuing antiplatelet therapy 7 days prior, new generation anticoagulants 48 hours prior, and warfarin until the international normalized ratio normalizes. A few hours before MRgFUS, the patient’s symptomatic medications (anti-Parkinson’s, anti-tremor, etc.) are also discontinued. It is recommended that the patient refrain from eating for at least 6 hours and drinking for at least 4 hours before undergoing MRgFUS.
On the day of the procedure, the patient arrives at the center with a relative or close person 2 hours before the start of treatment (fasting). A nurse inserts a peripheral venous catheter, applies compression stockings, and provides nausea and vomiting prevention (ondansetron 4 mg 30 minutes before the procedure, metoclopramide 10 mg intravenously may be administered to patients with ET). To prevent thrombotic complications, elastic stockings are applied. Due to the duration of the procedure (2–3 hours), we recommend using a diaper during the operation. Blood pressure is monitored in all patients, and glucose levels are monitored in patients with diabetes. Sixty minutes before the installation of the neurosurgical frame, the nurse applies lidocaine cream (if there is no allergic reaction) to the patient’s skin at the anticipated frame attachment sites.

Equipment calibration and application of the stereotactic frame
While the neurosurgeon and nurse prepare the patient on the morning of the surgery, the MRI diagnostics doctor, together with the MRI-technician, calibrates the MRgFUS machine before each operation. To do this, they perform a procedure on a special mannequin DQA. This procedure allows us to determine that the equipment is operating without errors, the thermal spot is formed at the correct size and at the right point. It is also important to ensure that the membrane to be used on the patient is defect-free. Figure 4.5 shows how the MRI-technician secures the mannequin inside the MRgFUS helmet.
The patient’s head is completely and thoroughly shaved 30 minutes before the treat-
nia. The necessity of thorough preparation is due to the fact that
what air bubbles that remain at the hair roots prevent
due to the possibility of cavitation during ultrasound.
On the scalp, four stereotactic fixation points are marked with a marker
stereotactic frame. Local anesthesia of the fixation points of the stereotactic frame was performed with ropivacaine.
The stereotactic frame for head immobilization is fixed to the skull with screws at four points. We recommend securing it in the supraorbital area on the right and left, as well as in the projection of the occipital protuberances at the back as low as possible, so that during treatment the surface area of the cranial part of the skull is as wide as possible; this allows a greater number of ultrasound waves to penetrate the cranial cavity and reach the necessary temperature indicators during the procedure.
Then place it on the head as low as possible (above the frame screws)
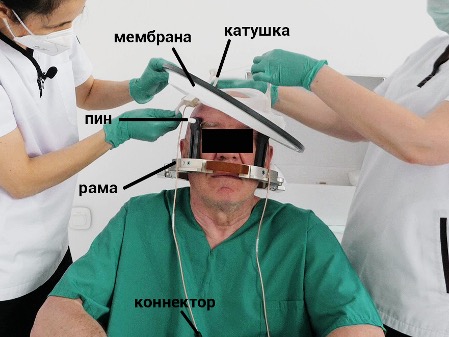
ется только что проверенная при помощи DQA Elastic disposable
silicone membrane (Fig. 4.6). This membrane contains a coil-
coils for MRI, and it is necessary to ensure that the coils are correctly
function. At the ends of the coils, deformations are eliminated even with
outer side. The membrane should fit snugly against the skin
covers to prevent water leaks. It is adjusted to fit dif-
Head measurement of the patient: for this, in the central part of the membrane, there is a-
Circular markings are used, cutting along which with scissors the fragment-
Elements of the membrane, you can change its size.
After this, the patient is placed on the table and is ready for the procedure.