MRgFUS Procedure
The novelty of MRI-guided focused ultrasound (MRgFUS) technology involves the continuous improvement of all intraoperative processes to reduce the risk of dangerous situations. In addition to the standard protocol provided by the equipment manufacturer, we have developed a series of special measures for this purpose.
MRI safety, item fixation, and climate control
Alongside standard measures prohibiting magnetic and electronic devices in the MRI room, we have proposed securing all additional equipment with climbing slings of strict length to loops fixed to the floor (Fig. 5.1). This is done to prevent oxygen concentrators, suction devices, or defibrillators from being brought into the Gauss zone in an emergency, as their entry into the magnetic field poses a serious risk of injury to both the patient and staff. At the same time, patient aspiration during the procedure is very dangerous and requires quick actions. If a suction system is not nearby, the patient would need to be removed from the table, which takes a lot of time because the patient is rigidly fixed to the focused ultrasound table. In such a situation, any delay is dangerous for the patient. In 6 cases, we needed to use a vacuum suction device. For this, the suction device secured with slings was brought to a safe distance from the table, and then the necessary sanitation was performed using an extended silicone tube. The slings are made as long as safely possible.
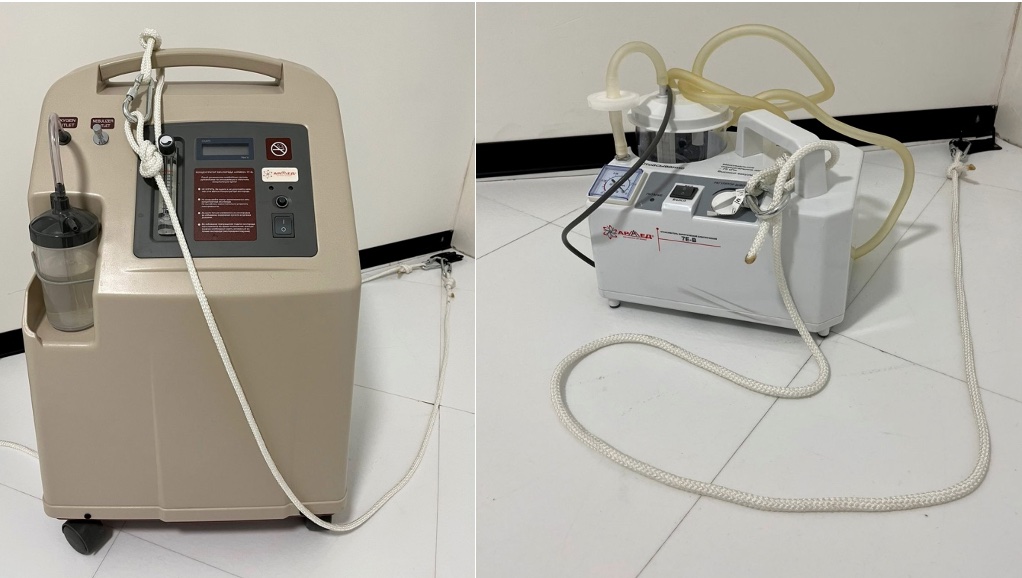
The placement of loops for securing equipment also deserves attention. To prevent slings from hanging in the air, it is better to place the loops on the floor so that when an employee brings equipment into the operating room, they do not create a tripping hazard for others, i.e., the rope should not lie across the path. In our case, the door loops are on the left, and the MRI machine is on the right of the door, so placing the loops on the left allows the equipment to be brought to the patient’s feet while ensuring staff have unobstructed access to the patient. The MRI room always maintains a certain air temperature to prevent equipment overheating. The patient lying almost motionless for several hours can lead to hypothermia. To prevent this, we recommend the patient wear thermal underwear and warm socks. During the procedure, the patient should be covered with a warm blanket or throw; hot water bottles should be kept ready if necessary.
Patient positioning
Proper patient positioning is a very important aspect that determines the comfortable and safe conduct of treatment.
The process of brain surgery using MRgFUS is conducted with the patient lying inside the MRI machine on a special table, which includes a helmet with ultrasound wave transmitters and a mechanical positioning block that moves and fixes the ultrasound source for precise targeting (Fig. 5.2). The patient table is also equipped with additional accessories that help keep the patient’s head in one position during treatment.
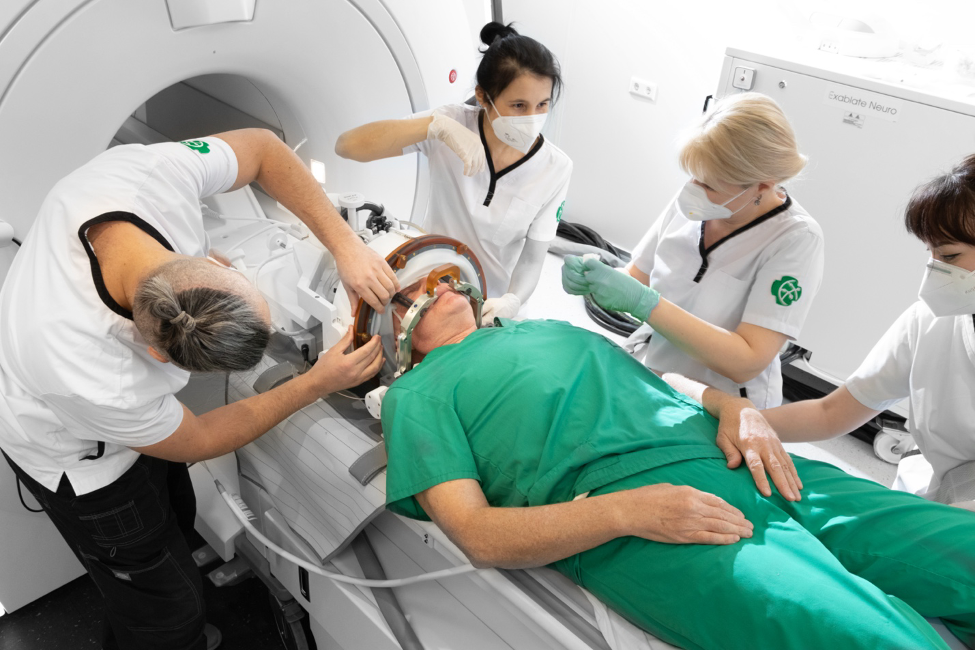
The patient’s head with a membrane and stereotactic frame is securely fixed to the attachment and to the MRgFUS table helmet with 1024 piezoelectric ultrasound wave generators. The membrane is connected to the transducer, and the space between the helmet and the membrane is filled with water cooled to 15–17°C and degassed. Using 3 guiding screws on the MRgFUS table, the helmet’s position relative to the head is adjusted so that the focus is no more than 0.5 mm from the target in all 3 planes. During positioning, it is necessary to smooth out any folds forming on the membrane as much as possible. If this is technically difficult, at least remove air bubbles by tapping motions. The importance of this manipulation is due to the fact that during treatment planning, these folds will be outlined by the computer, and the ultrasound transmitters in their projection will be excluded from the sonication process. The more folds there are, the more active elements will be turned off, and consequently, the lower the likelihood of achieving the planned temperature.
For the safety of the procedure, the patient is always given an emergency stop button in their hand. Right before the procedure begins, the neurosurgeon or neurologist once again explains when it should be used.
To protect against the noise effect of MRI, it is recommended to insert earplugs. Special soft-elastic cushions are placed under the lumbar spine and knee joints. Many patients cannot relax the cervical spine and continue trying to hold their head “in the air,” despite understanding the presence of firm fixation. To overcome this reaction, we recommend placing a thin rectangular foam pad between the neurosurgical frame and the skin: the sensation of pressure on the back of the neck and occiput allows the patient to relax their head.
Some patients begin to notice the onset of pain syndrome at the sites of attachment of the neurosurgical frame after positioning and water intake. If the intensity of the pain is above 5 points on the visual analog scale (VAS), a pain reliever is re-administered; if the intensity of the pain is less than 5 points, pain relief patches or anesthetic cream can be used.
In the case of relatives being in the operating room (the “open operating room” concept – see chapter 3), a chair, headphones, and a warm blanket are prepared for them in the MRI room.
Briefing and Checklist
Before the operation begins, readiness is checked by reading aloud our developed checklist. This is necessary to ensure the safety of the patient, the medical team, and relatives, as well as to properly check the readiness of the equipment and the correctness of the planned intervention side. After the briefing, the neurologist conducts a full examination of the patient on the table, recording it on video camera. This examination and video recording are very important for assessing the dynamics of changes at all subsequent stages of treatment using MRgFUS.
Our developed MRgFUS safety checklist .
Performing the MRgFUS Procedure
At the very beginning, the MRI-diagnosis doctor synchronizes the MRI equipment and the focused ultrasound table using localization scanning of the patient’s brain. Then, the MRI is controlled by the focused ultrasound workstation, performing a series of images in T1 mode, oriented in the coordinate system of the anterior and posterior commissures of the brain ( AC–PC). The obtained data is automatically loaded into the focused ultrasound console system.
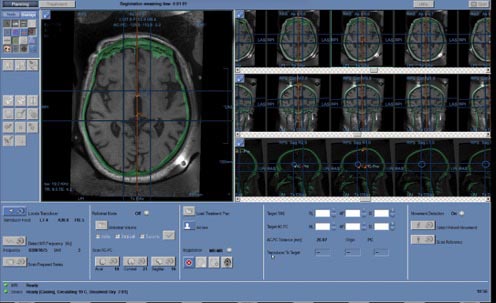
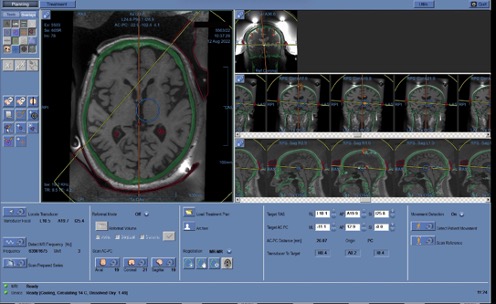
At the next stage, the neurosurgeon plans the treatment by combining previously taken CT images with MRI to determine the position of the target point, to which the transducer is moved using the mechanical positioning block of the focused ultrasound table. In Fig. 5.4, the skull bones are marked in green, overlaid on the black-and-white MRI image. The selection and calculation of the target ablation area (target) in the brain are carried out depending on the patient’s diagnosis. Before starting treatment, fiducial markers are placed on the hybrid image in brain areas with good structural contrast (marked with 5 crosses in Fig. 5.5). The markers are necessary to track possible head movement within the stereotactic frame. If movement is detected in the area of the fiducial markers, the system interrupts operation to avoid affecting the non-target zone. Five markers are placed in each of the 3 projections – axial, sagittal, and coronal.
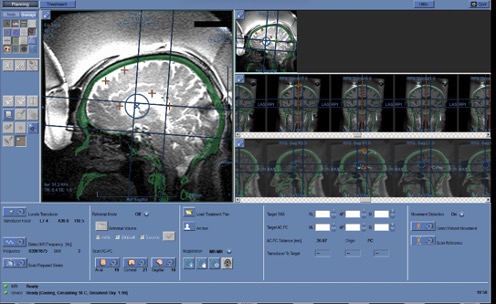
Frontal sinuses and other structures containing air, calcium, as well as folds of the MRI-guideded focused ultrasound membrane are marked on the planning console to exclude the passage of ultrasound waves. In Fig. 5.6, red circles indicate calcifications; cavities of irregular shape outlined by a red line are membrane folds; the midline is shown in orange; the blue circle represents the boundaries of the area where ultrasound exposure can be performed without mechanically repositioning the helmet.
During the MRgFUS procedure, the target is heated by ultrasound beams – this process is called sonication . The area of tissue exposed to temperature and the duration of exposure form an equivalent thermal dose, which determines the degree of thermal damage. Depending on the bone tissue density coefficient, the neurosurgeon calculates the energy delivered by the ultrasound exposure (J), power (W), and duration (s) of exposure to achieve the required temperature. During sonication, constant MRI measurement of temperature parameters at the target point is conducted every 3 seconds, with the transmission to the workstation screen of the size and shape of the thermal spot, temperature change graphs (maximum and average values), acoustic spectrum, and control reflecting cavitation during treatment. These graphs help the neurosurgeon monitor the temperature increase; if they see a deviation from expected parameters, they can stop the sonication by pressing a special button. The interval between sonications is determined by the cooling duration of the circulating water
The entire treatment process is divided into three stages:
- Calibration Align) – conducting sonications at temperature parameters from 40 о From up to 45ºC in three main dimensions, the neurosurgeon ensures that the system heats precisely at the selected point and the focus has a rounded shape.
- Focus Verification Verify) – sonication with the target point temperature reaching from 45 о Up to 50ºC to confirm that the impact on the selected point is accompanied by the required reversible clinical effect
- Treatment Treat low/high) – several sonications reaching a temperature of 55 о To up to 60 ºC to achieve the necessary and irreversible effect.
Calibration Align) – anatomical verification
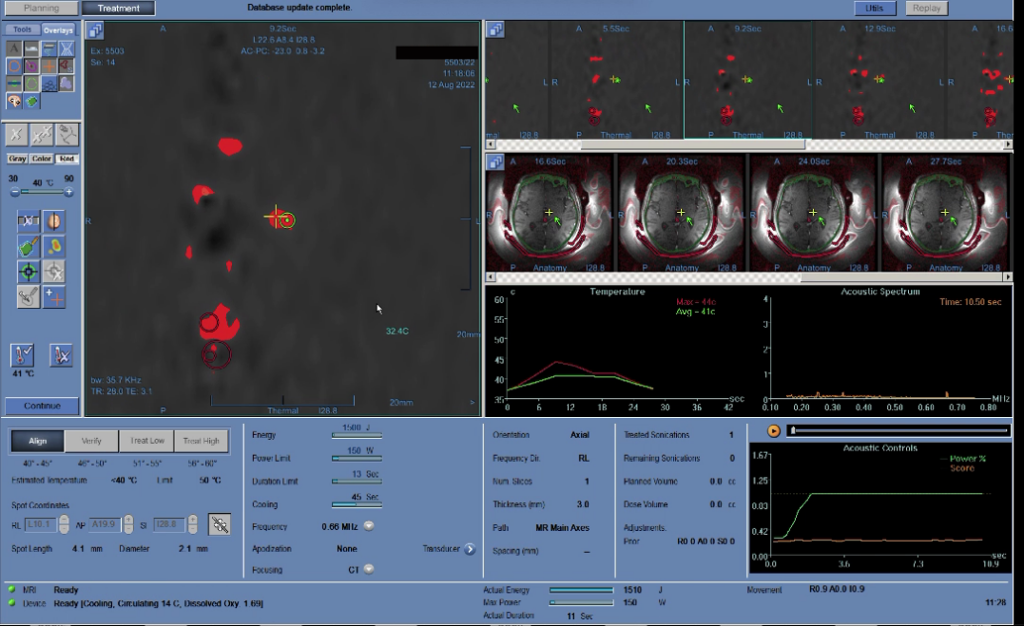
At the first stage, the neurosurgeon heats the target area to low temperatures (40–45ºC) and assesses the accuracy of the hit (displacement of the heated spot from the target). Thus, the device is calibrated in all three planes. In Fig. 5.7, the temperature graph during sonication is in the center, to the left on the MRI image is a red spot; the area marked with a cross is the heated section, and the nearby circle is the target. If the heated area is not at the target location, the neurosurgeon adjusts the aim and repeats the sonication. Corrections are made until any errors are eliminated.
Observing the temperature increase at the desired point, the neurosurgeon determines the parameters of the applied ultrasound energy
the energy (J), power (W), and duration (s) of exposure to achieve the required temperature he will use in the next two stages of treatment
Focus Verification Verify) – physiological verification
After the neurosurgeon ensures that the target is formed correctly, they move to the next stage—preliminary heating of the target area to a temperature that deactivates neurons without damaging them (45–50ºC) for a few seconds.
With such thermal exposure, a temporary reversible “shutdown” of brain cells occurs. During this short period, the neurologist assesses the patient’s response—changes in neurological status. At this stage, it is crucial to carefully evaluate the patient’s condition dynamics and the appearance of the slightest side reactions. A positive response is considered a reduction in the amplitude or frequency of tremor, decreased muscle rigidity and bradykinesia (in the case of Parkinson’s disease), and tension in the neck muscles (in the case of cervical dystonia). At the verification stage, it is difficult to achieve complete symptom disappearance due to low exposure temperatures, but an improvement of even 20–30% indicates the correctness of the chosen area.
If the expected improvement is not achieved during physiological verification or side effects occur, the target point or sonication parameters are adjusted. The neurosurgeon has sufficient opportunities to move the target point at this stage; however, each repetition of sonication causes reversible changes in brain tissue, complicating subsequent interventions. Sometimes, with a very high skull density coefficient (0.7–0.8) and thin cranial bones, even if the neurosurgeon has considered this, a significant thermal spot may form at the verification stage. Therefore, the choice of the target point should be made correctly and preferably on the first attempt.
The functional deactivation of neurons with ultrasound allows for modeling the future result of definitive therapeutic heating. Monitoring is conducted at each stage of the procedure to precisely know which area is being affected, what the thermal effects are, whether changes need to be made to the procedure parameters, and what the outcome will be immediately after the operation.
Treatment treat low/high) – therapeutic effect
After achieving the expected but temporary result during physiological verification, final treatment is performed by applying ultrasound waves to the target area of the brain, heating it to a temperature of 55–60°C. The MRgFUS procedure 97 Irreversible effects are repeated to expand the ablation area and consolidate the result.
After each sonication, a neurologist assesses the patient’s condition with video recording. The neurologist, neurosurgeon, and MRI diagnostician jointly make decisions on further steps based on the obtained data in a continuous consultation mode.
After the irreversible ultrasound treatment is performed, a final assessment of the treatment results is conducted through a neurological examination on the table and an MRI study of the brain. The MRI results and changes in the patient’s neurological status are discussed with the patient and their relatives. If a satisfactory result and sufficient size of the MRI focus are achieved, the treatment is usually concluded.
Neurological monitoring during the MRgFUS procedure
The team performing the MRgFUS procedure should always conduct a neurological examination of the patient between sonications. Different centers have adopted varying extents of patient examination. In some centers, complete neurological testing is not conducted during the MRgFUS procedure: for example, doctors may only check the effect of sonication on tremor or dystonia symptoms and then proceed to the next sonication, assessing further dynamics. This approach may be practical in terms of cost-effectiveness and the image of a “quick” neurosurgeon. However, the examination is needed not only to assess the effectiveness of treating tremor or other individual symptoms. An adequate amount of neurological testing during the MRgFUS procedure is very important for several reasons
1. Treatment Effectiveness. The observed elimination of tremor or other movement disorders after sonication indicates a correctly chosen target, allowing the neurosurgeon to continue treatment as planned or to complete it
2. Safety. A complete neurological examination can help identify unexpected neurological deficits (for example, due to atypical anatomy in a specific patient), which might be missed if limited to simple tests for tremor or muscle rigidity. For instance, if a mild paresis manifests in the leg during test sonication and the surgeon does not properly assess the condition of the lower limbs, the application of ultrasound in therapeutic mode could exacerbate this issue and potentially make it irreversible.
3. A complete neurological examination allows for a discussion with the patient and relatives about the further course of the operation. It may be advisable to draw the attention of the patient and relatives to neurological “findings” that appear during treatment, so that in the case of a suboptimal effect, the procedure can be concluded with their agreement to avoid more serious complications.
In our medical center, we conduct a complete examination between sonications according to a developed algorithm, which takes no more than 15 minutes and provides very important information to assess the balance of treatment effectiveness and safety and to stop in time. Experience with more than 200 MRgFUS procedures has allowed us to form and propose the following necessary and sufficient protocol for neurological examination between sonications.
1. Assessment of smell through a survey.
2. Vision assessment, preliminary visual field testing. Inquiry about visual acuity.
3. Tracking an object. Assessment of double vision. Examination for ptosis and strabismus.
4. Facial Sensitivity Assessment
5. Examination of the face at rest, detection of facial asymmetry, drooping of the corner of the mouth. Facial symmetry. Tests with forehead wrinkling, frowning, closing eyes tightly, showing teeth, puffing cheeks, stretching lips.
6. Approximate hearing assessment
7. Voice pitch (excluding dysphonia). Swallowing saliva.
8. Speech examination for dysarthria, difficulties in pronouncing words and sounds. Tongue protrusion (no deviation)
9. Muscle strength testing, examination of the range and speed of active movements. Raising arms up while lying down, flexion-extension in the joints, pronation-supination in the wrists, adduction-abduction of fingers. Range and speed of movements are assessed separately. For careful evaluation of even minor paresis, upper and lower Barre tests are used while lying on the back.
10. Muscle Strength Assessment. Evaluation of resistance to movement produced by the patient. The measurement is subjective, from 0 to 5 points, where 0 points – no movement; 1 point – slight movement; 2 points – movements possible without overcoming the force of gravity on the limbs; 3 points – the patient performs active movements but does not resist the doctor; 4 points – the patient resists but is weaker than the doctor; 5 points – full strength.
11. Study of passive movements and determination of muscle tone.
12. Study of Hyperkinesia Severity and Coordination. Examination of the upper and lower limbs at rest and during stress tests (subtracting from 100 by 7, reciting letters in reverse order, squeezing and releasing the contralateral hand). Finger-to-nose test for identifying intentional and kinetic tremor, pointing test. Heel-to-knee test. Examination of hyperkinesias (tremor and others) with arms raised above the head while lying down and with arms in front of the chest. Drawing spirals and lines. Test with a plastic spoon, test with a glass, test with a plastic bottle of water (allows assessment of tremor severity by liquid level fluctuations). Tests with additional objects, such as manipulating earplugs.
13. Checking the sensitivity of the skin in symmetrical points on the right and left sides of the body, from distal to proximal areas (the examination is conducted using plastic non-magnetic sticks or toothpicks). In our series of observations, 2 out of 200 patients showed a positive Barre test after trial sonication. This allowed us to change the target (shift the point of impact and conduct sonication more medially to avoid damaging the pyramidal pathways) and avoid complications. Additionally, several patients experienced numbness of the tongue tip or lower lip when the thalamus was targeted; in this case, the target was shifted forward to avoid damaging the sensory nucleus and prevent persistent functional impairment.
Therapeutic monitoring during the MRgFUS procedure
During the operation, the nurse monitors blood pressure. If it increases, antihypertensive drugs are administered. In case of headache, pain relievers are recommended (e.g., ketorolac 30 mg/ml), for the prevention of brain tissue edema – dexamethasone 4 mg/ml intravenously, and for the prevention and treatment of nausea and vomiting, ondansetron 2 mg/ml can be additionally administered. If necessary, humidified oxygen is supplied through nasal cannulas, and an aspirator is used in case of vomiting. As mentioned above, it is advisable to secure the equipment to the floor (contains ferromagnetics).
Procedure for Removal from the Focused Ultrasound Table
After completing the treatment, the patient remains on the table while the water drains from the system. The membrane is detached from the transducer, and the neurosurgical frame is removed from its attachment to the table. Only after this is the patient allowed to get up from the table and transfer to a transport chair-stretcher. In the dressing room, the silicone membrane and neurosurgical frame are removed, the attachment sites are treated, and bactericidal plasters are applied. The intravenous catheter is left in place for a few more hours for use if necessary.
Postoperative Stage
The patient remains under observation in the clinic for another 7–8 hours, with a follow-up MRI of the brain performed 2 hours after treatment and a repeat examination by a neurologist and neurosurgeon. The follow-up MRI is conducted in T2 mode FSE with 2 mm slices in the axial and coronal planes, as well as in modes SWAN, DWI, T1 FSPGR with 4 mm slices in the thalamus region in the axial plane. After MRI, depending on the severity of changes in the brain, dexamethasone is prescribed (intramuscularly or intravenously) to reduce the severity of edema. Procedure MRgFUS 101
The day after treatment, the patient, accompanied by a relative, returns to the clinic for a follow-up examination. A complete neurological examination is performed, and a follow-up MRI of the brain is conducted. On this day, further monitoring, regimen, the need for rehabilitation activities, and a schedule for subsequent consultations are discussed. Considering the non-invasive nature of the procedure, patients do not require a long recovery period, but it is recommended to follow a protective regimen without excessive stress for 2 weeks under the supervision of close ones.
In the postoperative period, patients take glucocorticosteroid medications for at least 2 weeks to prevent excessive swelling in the treatment area. All patients undergo follow-up brain MRI every 3 months according to a standard protocol.
Standardization of the Treatment Process Using MRgFUS
Due to the novelty of the MRgFUS method, staff preparation is required in addition to their basic training necessary for accreditation. Standardizing the process ensures consistent quality of care and provides the most reliable data for research.
Based on our experience, we have developed a clear standardized protocol for performing surgery using MRgFUS, presented in Appendix 2.
Target determination for MRgFUS
Two methods for determining the target for MRgFUS are proposed:
1) indirect targeting based on atlas and patient anatomy data on MRI brain scans, using the approximate location of the nucleus
2) direct tractographic approach, where targeting is personalized based on MR tractography ( Bruno et al., 2021).
Indirect targeting is a permissible approach to thalamotomy using MRgFUS, allowing for the immediate identification of the correct target in most cases. The direct tractographic approach is currently a valuable aid in assessing potential deviation in cases where an immediate clinical response is not achieved. Direct coordinates, for example, are proposed to be measured for the dentatorubrothalamic tract based on diffusion tensor MRI imaging.

Currently, the precise determination of the impact point is a major challenge. The issue is that the localization of the sonication target is conducted relative to the line connecting the anterior and posterior commissures ( AC–PC), what is the universal coordinate system for patients in functional neurosurgery. At the same time, MRI has its own coordinate system RAS, which is determined by the position relative to the isocenter of the device; additionally, the FUS station has its own coordinate system relative to the focal point. All three coordinate systems relate differently depending on the equipment’s position. To solve this problem, we created a special program in the language R, which allows recalculating coordinates depending on the positions of the zero point of the MRI and FUS station for more accurate target determination and reducing the possibility of error. Figure 5.8 shows an example of the relationship between these coordinate systems, where RAS – these are the coordinates relative to the MRI isocenter, transducer focal – coordinates of the FUS station, and AC–PC target – coordinates relative to the posterior commissure in the plane AC–PC.
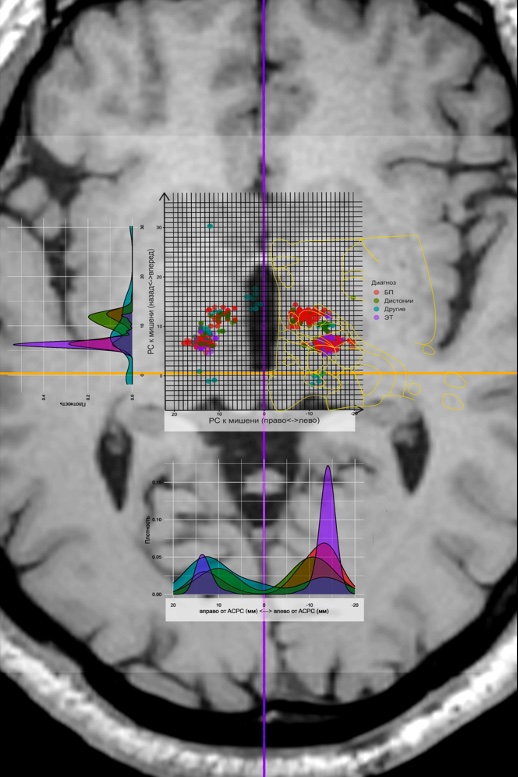
Figure 5.9 shows the treatment sonication map for all patients who underwent treatment using the MRgFUS method, on MRI in the axial plane passing through the line AC–PC.
Calculation of the Exact Target Temperature During Sonication
An important parameter for the effectiveness of treatment using MRgFUS is the temperatures reached at the point of impact. During sonication, the temperature increase occurs at different rates and depends on many parameters described by S. Gagliardo et al. (2019). The authors proposed calculating energy delivery curves for each treatment stage based on CT perfusion to determine ultrasound energy and ensure better control over the procedure’s outcome. However, they did not propose a clear formula for calculating heating energy.
As already mentioned above, during MRgFUS, the neurosurgeon uses specific parameters of energy, power, and duration of ultrasound waves to regulate the intensity of heating. During the first sonication at the calibration stage, the power is usually set at 250 W and the duration at 10 s, corresponding to an energy output of 2500 J. For subsequent sonications, the temperature setting is adjusted as follows. The neurosurgeon increases the power to such an extent that heating does not occur too quickly, as rapid heating can easily exceed the safe temperature limit for reversible effects. In other words, the power should be large enough to heat the area to the desired temperature within the set time, but the heating must be done at a rate that allows the neurosurgeon to stop the sonication in case of overheating. Excessive power can instantly overheat the target area of brain tissue. An unfavorable factor is that many patients feel the sonication: with increased power, headaches often intensify, and the patient may interrupt the treatment or even refuse to continue the operation. Too little power will not allow the area to be heated in the set time, but additional sonication may increase unnecessary tissue swelling—albeit temporary, but altering the neurological status at the time of the procedure.
At the same time, the duration determines the heating. More time allows more energy to be delivered to the area, increasing the heat. Too short a time won’t provide enough energy to heat the tissues, while an overly lengthy procedure may be poorly tolerated by the patient and lead to headaches. In this sense, reducing the treatment duration increases its tolerability for the patient. There are cases where the maximum power tolerated by the patient or generated by the device allows the area to be heated to the required temperature only by extending the sonication. The energy required to heat to the necessary temperature is calculated as the product of power and time. However, different patients require varying times to heat an area to a specific temperature.
Energy loss is influenced by KUPKT, or scull score, which is measured in values from 0 to 1. As the bone ultrasound transmission coefficient decreases, greater power and time are required to achieve the necessary temperatures. The bone ultrasound transmission coefficient is calculated using CT of the skull bones with the MRgFUS planning software package
In addition to the skull bones, water with varying concentrations of oxygen bubbles, which absorb ultrasound, stands in the way of the ultrasound. This issue is resolved by standardized cooling of the water, which sharply decreases oxygen solubility with a drop in temperature. The condition of tissues along the ultrasound path also alters its conductivity. There are other factors affecting the delivery, scattering, and reflection of energy. Therefore, using the KUPCT index as the sole guide is impossible (especially considering its inherent error).
The identification of clinically significant factors is useful for constructing a temperature model based on power, time, CT perfusion, and several other factors. Modern computer programs allow the use of complex algorithms and dependencies for model construction in the presence of multiple factors and uncertainty. To solve this problem, we created a special program that includes the following parameters: patient ID, sonication sequence number, achieved temperature (°C), power (W), sonication duration (s), early termination (1 – yes, 0 – no), measured energy (J), CT perfusion, CT machine used to determine CT perfusion Siemens, GE, Toshiba, Philips), data on the response to the first align-sonication, gender, and age of the patient. To determine dependencies, we built 2 models – linear and neural network.
The linear model was built in R package RStudio 2021.09.2 сборка 382, R version 4.2.1 (2022-06-23) regular team lm. As a result of the analysis of the obtained data, it was hypothesized that the linear model does not perfectly predict the temperature due to possible nonlinear dependencies. A neural network-based regression solution could be suitable for building the model.
Neural network model was created in R package RStudio 2021.09.2 сборка 382, R version 4.2.1 (2022-06-23), what were open libraries for production-level machine learning used for TensorFlow. Details of the neural network model construction and the analysis conducted with its help can be provided upon request.
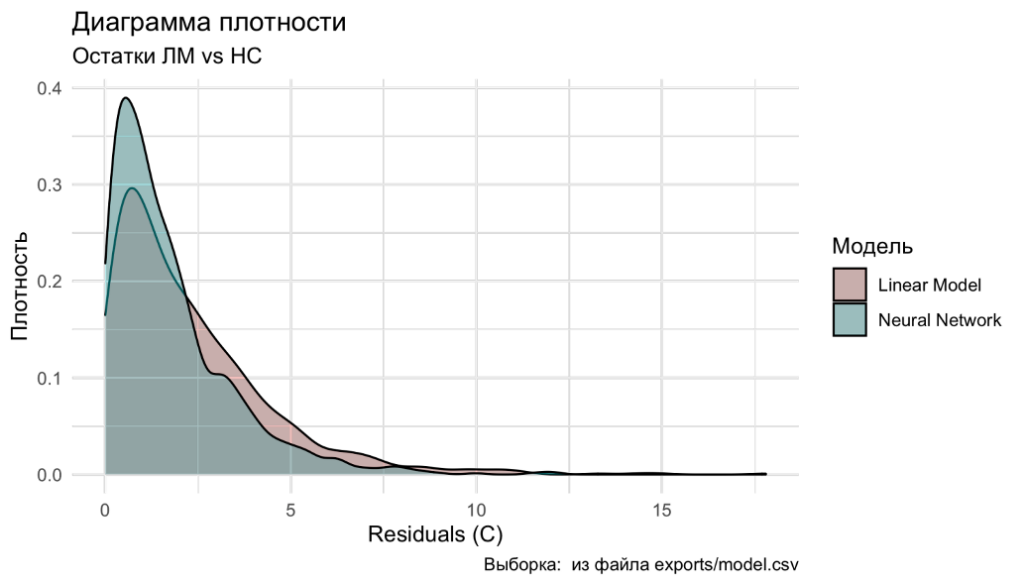
When analyzing the data models, the maximum prediction error for the linear model was 17.8°C, and for the neural network model, it was 12.1°C. Thus, the neural network model for temperature calculation proved to be better. Figure 5.10 shows the density distribution diagram of the residuals, where it is evident that the neural network model is closer to zero error. The corresponding calculations can be practically implemented during the planning and execution of treatment using MRgFUS with the table we proposed (Figure 5.11).
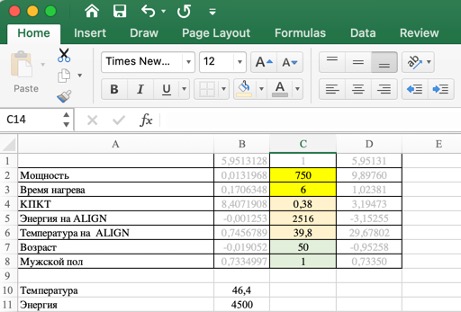
Thus, the temperature of the target during ultrasound heating is influenced not only by power, energy, and time as sonication parameters but also by the CT perfusion index, as well as the patient’s gender and age. The parameters of the first sonication during the calibration stage and the response to it are useful for predicting the parameters of subsequent sonications. The linear model allows predicting the temperature with a mean absolute error MAE) = 2,78O S, and the coefficient of determination R2 = 0,71, whereas the neural network – with a mean absolute error of 1.93 O S and R2 = 0,76. A disadvantage of the linear model is its lower accuracy, but it is easily accessible and convenient in the operating room, as it does not require additional software.
Features of the Cohort of Patients We Operated On
The principles of work organization and action algorithms outlined above were successfully applied by our team at the first center in the Russian Federation for treatment using MRgFUS: from 2020 to 2023, 86 patients with PD (including 82 with predominant tremor and 4 with akinetic-rigid form of the disease), 37 patients with ET, 18 patients with various forms of dystonia, as well as 1 patient each with neuropathic pain and hypothalamic hamartoma, were treated at the V.S. Buzaev International Medical Centre (Ufa). From the first day, the work of the MRgFUS center was conducted under the scientific and methodological guidance and in close cooperation with the Research Center of Neurology and the National Society for the Study of Parkinson’s Disease and Movement Disorders.
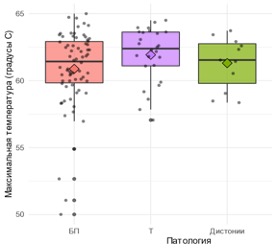
The age of operated patients (Fig. 5.12) in the main groups with movement disorders differed statistically significantly ( p < 0,001 by the Kruskal-Wallis criterion): the average age of patients with PD was 63 [55; 70] year, patients with ET – 54.50 [36,25; 64,75] year, patients with dystonia – 46.0 [39,0; 53,5] years. There were no age differences between men and women
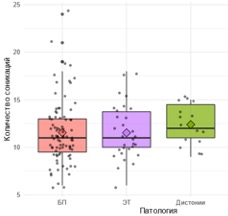
The median duration of the operation (Fig. 5.13) was 98.7 [74,7; 132,6] min, including in the PD group – 97.2 [73,6; 126,4] min, in the ET group – 96.1 [72,8; 126,4] min, in the dystonia group – 117.1 [79,1; 139,2] min, differences are statistically insignificant (p = 0.53). The median number of sonications (Fig. 5.14) was 11 [10; 14], with slight variations between groups. The maximum temperature of the sonication focus during the operation (Fig. 5.15) was 61.6 [59,8; 63,0]°C, maximum sonication energy (Fig. 5.16) – 20,035 [14 030; 32 330] J, there were no significant differences between the groups.



International Internet Operating Room: Application of MRgFUS in Telemedicine Mode
Usually, for the theoretical training of doctors and the development of their manual skills, special training programs from the manufacturer are used. However, the best practice is to have a dedicated specialist for supervision and consultation during the first operations ( Broering et al., 2020). Many manufacturers do not allow their treatment programs to be initiated without authorized assistance and supervision, especially when it involves new technologies. According to the manufacturer’s standard policy, it is advisable to conduct the first 20–25 operations under the supervision of a specialist, after which the team receives “solo” status, i.e., permission to perform the procedure independently.
Travel restrictions during the pandemic COVID-19 led to a large-scale transition of educational programs at various levels to online digital and distance learning. However, the implementation of such approaches for entirely new, complex technologies may be associated with serious and quite understandable difficulties. The initiation of the practical work of our MRgFUS center coincided with the peak of the pandemic: in April 2020, the equipment installation was completed InSightec ExAblate 4000 (in the surgery waiting list were 32 patients), but at that moment it became clear that the personal arrival of experts from the manufacturer was impossible due to quarantine restrictions. In this regard, we were the first in the world to develop and implement a treatment program using MRgFUS with international remote online monitoring, assessing the effectiveness and safety of this telemedicine approach, as well as the immediate results of the operations performed
The study included the first 94 patients who underwent thalamotomy using MRgFUS in our center, including 27 patients with ET and 67 patients with PD (tremor-dominant phenotypes). Under online control, 38 patients received treatment and without it (group ” cоло”) – 56 patients. The study began on May 5, 2020, and continued for 1 year and 9 months. The center’s team was certified to perform independent operations (” colo”) after performing 38 MRgFUS procedures.
To present the results we obtained both within this study and in further independent work (see chapters 6–8), it is necessary to introduce a number of defining terms. By “attempt,” we mean the situation when a patient is placed on the MRgFUS table and the first ultrasound exposure is performed. We define the “success” of the procedure as a 30% reduction in the severity of tremor according to CRST and/or by UPDRS (part III) on the side contralateral to the performed operation
We also used the following subjective scale: “excellent” effect – complete elimination of movement disorder symptoms and achievement of all major surgical goals; “good” effect – only minor symptoms remain after MRgFUS treatment; “satisfactory” effect (“compromise”) – a certain positive result was achieved during the procedure, but it was stopped because the expected risk of complications from continuing ultrasound exposure was higher than the expected benefit. These 3 categories are considered “successful.” A “failed” result was recorded if the intervention did not significantly impact the severity of the disease’s clinical manifestations. In no case of MRgFUS application did we observe a deterioration in the patient’s condition.
Telemedicine Remote Expert Monitoring
The world’s first telemedicine implementation of the MRgFUS technology took place on May 5, 2020. We ensured the virtual presence of company experts InSightec Paula Regg (United Kingdom), Tessa Case (Spain), Daniil Molchanov (Russia), as well as project curators – President of the National Society for the Study of Parkinson’s Disease and Movement Disorders, Academician of the RAS S.N. Illarioshkin, and Chief Neurosurgeon of the Ministry of Health of the Republic of Bashkortostan, Doctor of Medical Sciences Sh.M. Safin during the first procedure. Before the first treatment session using MRgFUS, a two-week training process took place with preliminary discussion of each patient to ensure the most appropriate selection.
To organize the first telemedicine treatment using MRgFUS, in addition to the traditional organization of a video conference with all participants, it was necessary to solve 3 tasks.
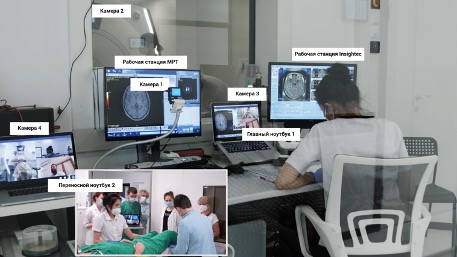
Firstly, to ensure safety and constant visual monitoring of the patient’s condition, we needed to provide secure video streaming (in real-time) of the patient’s condition in the MRI room during treatment. Additionally, foreign experts were required to evaluate the results of neurological testing conducted by our team of neurologists to assess treatment effectiveness. The challenge was that due to the design features of the MRI room and the constant magnetic field, it was impossible to place cameras directly inside the room. We conducted 3 procedures using a webcam Microsoft, mounted on the ceiling of the MRI room. During the 3rd operation on MRI thermometry, intense noise began to occur, so the only solution was to remove the camera from the MRI room and install it in front of the observation window (camera 2 in Fig. 5.17). We streamed the video feed from the cameras into a video conference Microsoft Teams. A portable laptop was used during neurological tests MacBook Pro with a built-in camera (portable laptop 2 in Fig. 5.17)
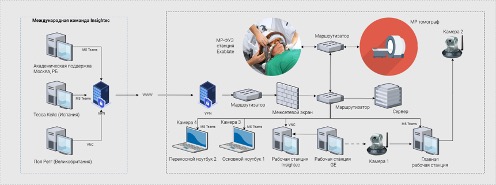
Secondly, it was necessary to organize the transmission of telemetry from the equipment InSightec to the international team of engineers so they could observe how the chosen treatment strategy aligns with the equipment settings and adjust them if necessary to achieve the desired outcome. No one in the world had such experience. After analyzing all available technologies, the most promising technologies were selected Virtual Network Computing (VNC) и Port Address Translation, with which we were able to organize secure remote connection sessions for experts located during treatment in the UK, Spain, Israel, and St. Petersburg (Fig. 5.18)
Thirdly, it was necessary to ensure the transmission of the patient’s MRI images so that the international team could assess the effectiveness of the treatment and adjust the strategy with us. Since the software and hardware system General Electric represents a fully closed system, we did not have the opportunity to connect to it directly or use additional technologies for intercepting and transmitting images (similar to converters HDMI в USB). To solve this task, we used a camera directed at the MRI monitor, and the video stream from the camera was transmitted to a video conference for all participants (camera 1 in Fig. 5.18). This image was not used for making clinical decisions but only provided the ability to manage settings on the console by the MRI-technician. The highest quality MRI image in real-time, as usual, was transmitted to the InSightec ExAblate 4000 (workstation InSightec in Fig. 5.18) according to the device protocol InSightec. Then the image was broadcast from the console InSightec protocol experts VNC. Microsoft Teams used as a platform for video conferencing among all participants (see Fig. 5.18). The choice of this technology was due to the corporate standard InSightec, and its functionality met all necessary requirements. We used 4 cameras: camera 1 for the MRI console, camera 2 for the MRI room, camera 3 for the neurosurgeon, camera 4 for neurological tests and temporary close-ups inside the MRI room. Portable laptop 2 with camera 4 was brought into the MRI room only when the MRI machine was not scanning (see Fig. 5.18)
Neurosurgeon, radiologist, and MRI-technician communicated with experts using the main laptop 1 (see Fig. 5.18). The workstation console InSightec thanks to the use of the protocol VNC allowed the neurosurgeon in Ufa to operate the MRgFUS device and enabled the use of the mouse cursor in collaboration with experts from the operating table manufacturer. The MRI workstation console was controlled by a radiologist and an MRI specialist.
Thus, solving these three tasks made it possible to ensure the necessary level of patient safety and conduct the world’s first telemedicine treatment using MRgFUS.
Comparison of Surgery Outcomes Performed with Telemedicine Monitoring and Independently
In total, thalamotomy using the MRgFUS method under international online supervision was performed on 38 patients with tremor (11 patients with ET and 27 patients with PD), and independently we operated on 56 patients (16 patients with ET and 40 patients with PD) (Fig. 5.19). There were no significant age differences between patients in the specified groups.
In the ET group with telemedicine support, the procedure was successful in 10 (81.8%) out of 11 patients, while in the self-treatment group, all 16 patients (100%) were successful. No recurrence of tremor was recorded in either group. When evaluating the treatment results by CRST (Fig. 5.20) No statistically significant differences were found between the groups of self-treatment and treatment with telemedicine support
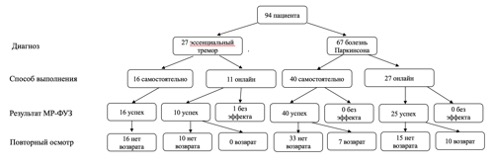
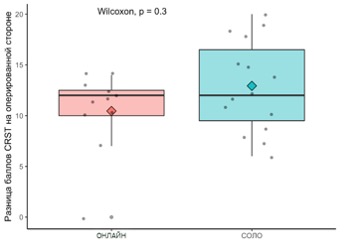
In the group with telemedicine support, the procedure was successful in 25 (92.6%) out of 27 patients, while in the self-treatment group, all 40 patients (100%) were successful. A partial recurrence of tremor within 1 year was observed in 10 (40%) out of 25 patients in the telemedicine support group, and in 7 (17.5%) out of 40 in the self-treatment group, with statistically significant differences ( p = 0,04 by the χ2 criterion). When comparing the condition of operated patients by UPDRS (part III – motor functions) it was found that the improvement achieved in the self-treatment group was statistically more significant ( p = 0,0026 by the Wilcoxon criterion) than in the treatment group with telemedicine control (Fig. 5.21)
Thus, we were the first to demonstrate that remote expert monitoring during treatment with MRgFUS is effective and safe. For ET and PD, the treatment outcomes in the groups with remote telemedicine monitoring and independent treatment were generally comparable in terms of achieved improvement, complication rates, and long-term results (tremor recurrence). Interestingly, in the PD group, the percentage of symptom reduction was significantly higher in the independent treatment group than in the telemedicine monitoring group; this can be explained by the fact that during online training, experts were restricted by a strict protocol with clearly defined targets, whereas in the process of independent treatment, we had the opportunity to target other areas with MRgFUS not included in the manufacturer’s protocol (for more details, see Chapter 7)

The method we developed for launching a telemedicine remote treatment program with MRI-guideded focused ultrasound under online supervision can be recommended for implementation by other centers in the process of mastering this technology. The world’s first international publication on this topic was presented by us in 2023 (Buzaev et al., 2023) and has already received very favorable feedback. Given the rapid development of the method and the emergence of new MRgFUS centers worldwide and in our country, the experience gained is very valuable and timely. Since various telemedicine approaches are currently in the spotlight, some of the developments and technological solutions we have made can be transferred from neurosurgery to other clinical specialties.